VOILAAAA!
My friend "Banban" came back to see me with his Pro equipment! no less than 50K € of instrumentation!
We settled in the basement in the boiler room.

On the left, a Pelletier effect air dryer for working with dry gases
In the middle VOC Analyzer (Volatile Organic Compounds) with its burner that carbides with hydrogen + Helium, to quantify the amount of suspended particles (unburned) and check the quality of combustion in ppm equivalent CH4 on wet gas only
On the right, a HORIBA analyzer able to give us precise measurements of NOx Chimieluminescence, SO2 infra red, CO Infra red, CO2 infrared, O2 Paramagnetic
Behind, the calibration gases.
What strikes at first glance is the almost zero amount of VOC measured whatever the following settings while the measurements are stable around the 400 / 500 ppm equivalent CH4 on wet gas for a traditional burner:
Here are the readings of the last adjustment of the boiler, the one which "cleans" the boiler

Not bad for a quick setting
 WHAT WE CAN ABOVE AND ALREADY AFFIRM, IT IS THAT THE RE-CIRCULATION OF THE WATER VAPOR FROM THE COMBUSTION OF THE FUEL ALLOWS TO DIVIDE THE ORGANIC POLLUTION (carbon and soot) BY 400 !! BE ABOUT NIL, IT IS NOTHING!
WHAT WE CAN ABOVE AND ALREADY AFFIRM, IT IS THAT THE RE-CIRCULATION OF THE WATER VAPOR FROM THE COMBUSTION OF THE FUEL ALLOWS TO DIVIDE THE ORGANIC POLLUTION (carbon and soot) BY 400 !! BE ABOUT NIL, IT IS NOTHING!
For the history and validation of the 950 threshold of flame T ° for the production of CO, below increase of the air flow:

With the beautiful blue flame you know: with this assembly Blue = CO because too cold flame, here 270 ppm CO!

INCREASE THE AIR FLOW AND THE CO INCREASES !! CQFD note how VOCs (soot) increase with CO production
 THIS IS OUR BEST SETTING
THIS IS OUR BEST SETTING

WITH A FLAME THAT IS NOT TOTALLY BLUE
WHERE IT IS NECESSARY TO ADJUST WITH A CO-ANALYZER
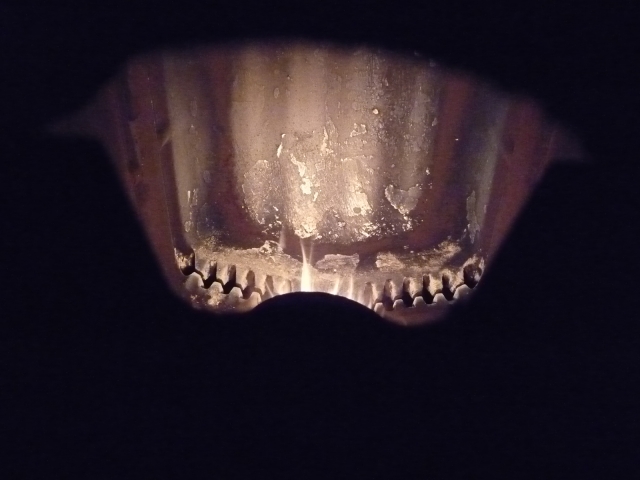
The flame temperature is not homogeneous, and for this last adjustment, the T ° is greater than 1000 ° C but with an outer layer less than 950 ° C because of the boundary layer due to recycling
 For comparisons, here are the previous measurements made before adding my assembly on the burner, edifying no?
CO divided by 9 !!
For comparisons, here are the previous measurements made before adding my assembly on the burner, edifying no?
CO divided by 9 !!
Carbon and soot divided by 400 !!
NOx divided by 20 !!

DEMONSTRATION IS MADE THAT WATER VAPOR INJECTION IS HIGHLY BENEFICIENT TO THE COMBUSTION OF GASOIL
THEN, GO TO VEHICLES WHEN?
perfect combustion reaction GO (nitrogen is not present)
C2H16 34 + = 49 2 O32 CO2 + 34 H2O
With this and in view of the results obtained, it is not easy to calculate the percentage of water to inject knowing that 50% of the gases are recycled in the boiler

And There you go! My old oil burner is less polluting than the last gas boilers !!
Except that the boiler is not up to the height ... the heater is not super tight, and the price of fuel at this time, will have to do something

but OK,
the primary goal was to demonstrate the effectiveness of the water vapor, succeeded no?
I claim and I sign: P. MALOCHET





