we start by charging FAP and cacalytics that I find too expensive and not efficient
and you want to add an even more expensive layer with the hybrid
water and limestone absorption is a really cheap way ... can be a little more bulky than the catalytic converter, but incomparably cheaper!
and easy to maintain at no cost ... when the limestone is dirty you empty it in your garden and put a peel of new gravel instead
NOx make nitrates with limestone which can be used as fertilizers
and this system is also a radical solution for wood heating
when it is cold a car equipped with this system smokes a lot! but it's mostly steam! and an exhaust pot that pisses water on the floor
Impunity of a killer: Diesel diesel
- chatelot16
- Econologue expert

- posts: 6960
- Registration: 11/11/07, 17:33
- Location: Angouleme
- x 264
chatelot16 wrote:we start by charging FAP and cacalytics that I find too expensive and not efficient
and you want to add an even more expensive layer with the hybrid
No, I want to remove the layer of soot in town.
We can make uncomplicated hybrids. I made one personally.
I do not know if hard water filters NOx so well and even VOCs, in general, VOCs, as carbon chains are rather oily and hydrophobic (especially unburnt Diesel). NOx dissolves very little in water.
What works well is the wet tissue, because the water clogs the mesh of the tissue down to the molecular level and blocks all the large particles of soot, which eventually get caught in the fibers.
On the other hand, for all polluting and highly volatile gases such as NOx, CO, and other VOCs originating from the FAP, there is not much to do, that is why I say that combustion must be suppressed as much as possible. city by using the hybrid, or even 100% electric.
On the contrary, in a city or open motorway, gas is not a problem (big dilution and few people around), that's good because combustion is absolutely necessary for autonomy.
0 x
- chatelot16
- Econologue expert

- posts: 6960
- Registration: 11/11/07, 17:33
- Location: Angouleme
- x 264
N02 is not only soluble in water: it is completely decomposed to make nitric acid, which hastens to make calcium nitrate with limestone
NO is simply soluble in water which could only absorb a limited amount, but also as long as the water contains limestone it can absorb it easily
limestone is essential otherwise water once saturated would release everything without being used for anything
there are a lot of products that are hydrophobic, but what counts is especially the brutal cooling in contact with water, which does not make fine particles
the worst is the volatile product which condenses in the air into very fine particles
NO is simply soluble in water which could only absorb a limited amount, but also as long as the water contains limestone it can absorb it easily
limestone is essential otherwise water once saturated would release everything without being used for anything
there are a lot of products that are hydrophobic, but what counts is especially the brutal cooling in contact with water, which does not make fine particles
the worst is the volatile product which condenses in the air into very fine particles
0 x
- chatelot16
- Econologue expert

- posts: 6960
- Registration: 11/11/07, 17:33
- Location: Angouleme
- x 264
Remundo wrote:On the other hand, for all polluting and highly volatile gases such as NOx, CO, and other VOCs from the FAP, there is not much to do
the current catalytic converter obliges to make a compromise between unburnt and NOx ... with limestone radical solution against NOx one can choose poor mixture and high compression ratio without EGR to make no unburnt and CO, but if that makes a lot of NOx
it is also interesting for petrol engines, whose performance is deliberately degraded so as not to burn too much NOx and leave enough unburnt to heat the catalytic converter ... with the complication of the lambda probe
with a good absorption of NOx, we can choose a poor mixture and have no unburnt ... and no more need for the catalytic converter which disperses its precious metals ... which we will find in a few years as bad as the lead and asbestos
0 x
yes, lean mixture with large compression ratio, it is the best performance, both combustion and thermodynamic. But it promotes NOx
In Diesel, it lends itself very well. Not in petrol (rattling / self-detonation)
If you think that the dissolution is effective, you would need a sort of bubbler of hard water to drain regularly ...
In Diesel, it lends itself very well. Not in petrol (rattling / self-detonation)
If you think that the dissolution is effective, you would need a sort of bubbler of hard water to drain regularly ...
0 x
So to complete, my fears are confirmed, and the idea of Chatelot too ...
SourceNOx Removal Technologies
But NO slowly turns into NO2
Then NO2 in water is converted to acid according to 2 processes ... self-oxidation or by hydrolysis.
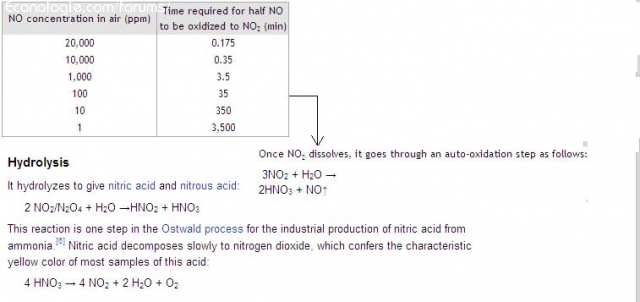
source Wikipedia
Not sure that the kinetics of these reactions are fast enough to swallow the gases of an Hdi of 140 hp. Unless you have a very large bubbler + acidic water management
On the other hand, industrially, 3 NO2 + H2O -> 2 HNO3 + NO is used in the Ostwald process for obtaining nitric acid.
@+
NO is a colorless gas with virtually no water solubility
[]
NO2 has a very low solubility in water. However, NO2 will slowly dissolve
SourceNOx Removal Technologies
But NO slowly turns into NO2
Then NO2 in water is converted to acid according to 2 processes ... self-oxidation or by hydrolysis.
source Wikipedia
Not sure that the kinetics of these reactions are fast enough to swallow the gases of an Hdi of 140 hp. Unless you have a very large bubbler + acidic water management
On the other hand, industrially, 3 NO2 + H2O -> 2 HNO3 + NO is used in the Ostwald process for obtaining nitric acid.
@+
0 x
- chatelot16
- Econologue expert

- posts: 6960
- Registration: 11/11/07, 17:33
- Location: Angouleme
- x 264
N02 does not dissolve in water, it forms nitric acid and leaves NO in rab which it can dissolve, and transform into NO2 when it has time ...
when you want to use pure water to make nitric acid it's quite complicated because the nitric acid evaporates: so you need a methodical circulation and a large exchange surface ... but when there is limestone in the water, the acid is immediately neutralized and does not prevent the absorption of the following NO2
the instantaneous production of nitric acid when NO2 falls on wet mucous membranes is what makes this gas dangerous: the quantity produced by an engine is very small: if there is a sufficient volume of water to cool the gas exhaust it is easily able to absorb all NO2
this water turns in a closed circuit, with a pump and a large radiator to cool it: therefore the water produced by combustion condenses there and the circuit never stops overflowing: it is this overflow which causes the nitrate calcium and other pigs absorbed in water
this water circuit sprinkles a limestone gravel filter, which allows both to saturate the limestone water and neutralize the acid, and to serve as a wet filter or the exhaust gas will lose its particles
the advantage of this wet filter is that it does not try to transform large particles into multiple fine particles by passing them in force through fine porosity
the oxidation of NO to NO2 is quite slow but it is not stuffed: the water remains in the system for a long time: the solubility of NO in water is low but sufficient
the abundance of CO2 in the exhaust allows the water to dissolve a large amount of limestone: the water which overflows is a real petrifying fountain, when this water loses its CO2 to the air it forms solid limestone which traps all the particles mixed with water, instead of letting them go into dust as if it were pure water
the overflowing water looks like black ink, which makes black limestone stalactite
when you want to use pure water to make nitric acid it's quite complicated because the nitric acid evaporates: so you need a methodical circulation and a large exchange surface ... but when there is limestone in the water, the acid is immediately neutralized and does not prevent the absorption of the following NO2
the instantaneous production of nitric acid when NO2 falls on wet mucous membranes is what makes this gas dangerous: the quantity produced by an engine is very small: if there is a sufficient volume of water to cool the gas exhaust it is easily able to absorb all NO2
this water turns in a closed circuit, with a pump and a large radiator to cool it: therefore the water produced by combustion condenses there and the circuit never stops overflowing: it is this overflow which causes the nitrate calcium and other pigs absorbed in water
this water circuit sprinkles a limestone gravel filter, which allows both to saturate the limestone water and neutralize the acid, and to serve as a wet filter or the exhaust gas will lose its particles
the advantage of this wet filter is that it does not try to transform large particles into multiple fine particles by passing them in force through fine porosity
the oxidation of NO to NO2 is quite slow but it is not stuffed: the water remains in the system for a long time: the solubility of NO in water is low but sufficient
the abundance of CO2 in the exhaust allows the water to dissolve a large amount of limestone: the water which overflows is a real petrifying fountain, when this water loses its CO2 to the air it forms solid limestone which traps all the particles mixed with water, instead of letting them go into dust as if it were pure water
the overflowing water looks like black ink, which makes black limestone stalactite
0 x
Hello,
indeed, aqueous H + NO3- reacts with carbonates CO3_2-
2H + + CO3_2- -> CO2 + H2O
we therefore ideally have a solution of neutral calcium nitrate, which can be rejected in nature without much damage. It is in particular a fertilizer / fertilizer.
Have you tried this technique on your generator sets?
indeed, aqueous H + NO3- reacts with carbonates CO3_2-
2H + + CO3_2- -> CO2 + H2O
we therefore ideally have a solution of neutral calcium nitrate, which can be rejected in nature without much damage. It is in particular a fertilizer / fertilizer.
Have you tried this technique on your generator sets?
0 x
- chatelot16
- Econologue expert

- posts: 6960
- Registration: 11/11/07, 17:33
- Location: Angouleme
- x 264
I had tested on a group of 2000W with LPG which in summer spread a disagreeable and irritating odor, when the exhaust gas at the exit of a too large exhaust pot is no longer hot enough to go up and stupidly builds up around
a large sprinkling with water of the interior of the exhaust made well disappear the irritating odor, which I attributed to NO2 but without having made the least measurement to be on
the problem is that my sprinkling of water made my scrap rust too quickly ... so you have to redo everything in plastic
no need to do stainless steel, because thanks to the water flow everything is at low temperature
I will especially make this system for a wood stove: it will be much more useful: radical elimination of any smoke ... total recovery of the heat lost in the smoke: evacuation of the smoke by a very cold plastic pipe: therefore possibility to put a wood stove anywhere even if there is no real chimney
a large sprinkling with water of the interior of the exhaust made well disappear the irritating odor, which I attributed to NO2 but without having made the least measurement to be on
the problem is that my sprinkling of water made my scrap rust too quickly ... so you have to redo everything in plastic
no need to do stainless steel, because thanks to the water flow everything is at low temperature
I will especially make this system for a wood stove: it will be much more useful: radical elimination of any smoke ... total recovery of the heat lost in the smoke: evacuation of the smoke by a very cold plastic pipe: therefore possibility to put a wood stove anywhere even if there is no real chimney
0 x
Hello Remundo,
?????... However...:
post202958.html # 202958
Remundo wrote:Besides, those who make Gillier Pantone notice much less polluted gases, without a doubt that water plays a role in fixing particles.
?????... However...:
1) H2 creation reaction with carbon soot from unburnt with water: C + H20O + heat = CO + H2
Consequence: very good improvement in combustion, on the one hand because we reduce the soot in the cycle, on the other hand thanks to the H2 created which burns extremely well!
Developed in detail here: understanding injection water / doping motor-al-water-thermodynamic-t4883.html
post202958.html # 202958
0 x
Go back to "Fossil energies: oil, gas, coal and nuclear electricity (fission and fusion)"
Who is online ?
Users browsing this forum : Majestic-12 [Bot] and 238 guests

